THE CELL BIOLOGY OF GLIA IN DEVELOPMENT AND DISEASE
Glia are a frontier of neuroscience, and overwhelming evidence from the last decade shows that they are essential regulators of all aspects of the nervous system. The Zuchero Lab aims to uncover how glial cells regulate neural development and how their dysfunction contributes to diseases like multiple sclerosis (MS) and in injuries like stroke.
Although glia represent more than half of the cells in the human brain, fundamental questions remain to be answered. How do glia develop their highly specialized morphologies and interact with neurons to powerfully control form and function of the nervous system? How is this disrupted in neurodegenerative diseases and after injury? By bringing cutting-edge cell biology techniques to the study of glia, we aim to uncover how glia help sculpt and regulate the nervous system and test their potential as novel, untapped therapeutic targets for disease and injury.
ONGOING RESEARCH
What is the mechanism of myelin wrapping? Why does regeneration of myelin fail in diseases like MS?
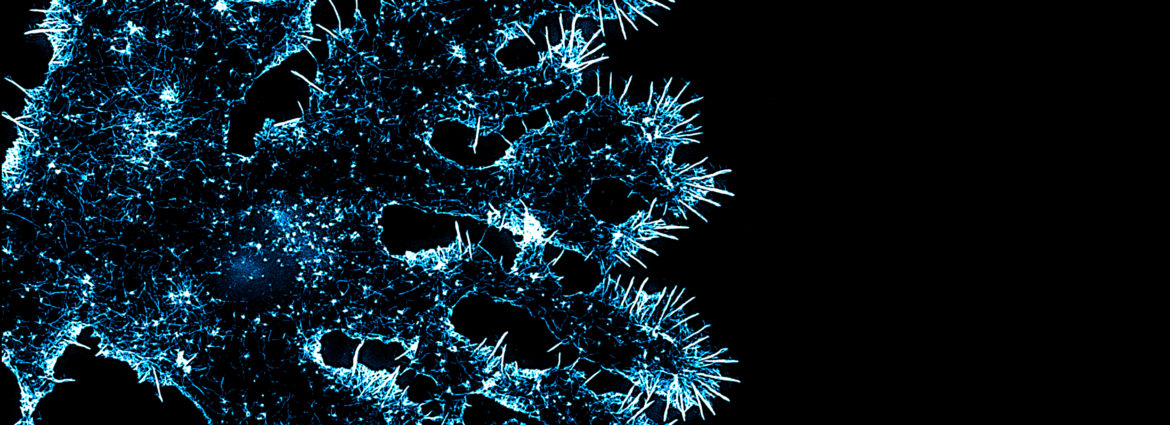
The spiral wrapping and compaction of OL processes around axons is a complex and mysterious process in cellular neuroscience. Myelin wrapping is required for fast nerve conduction, and its failure in diseases like MS, causes disability in patients. We showed previously that disassembly of the OL actin cytoskeleton is the key trigger to initiate wrapping after an OL process first engages and ensheaths the axon. This finding was completely unexpected and opens up many new questions: How does actin disassembly induce wrapping? Is actin disassembly perturbed in diseases of myelin, like MS? We are using a combination of powerful approaches to address these questions, including superresolution microscopy and live cell imaging of glia in vivo and in culture. Longer term goals of our lab will be to use what we learn about myelination during development to test whether the cell biological pathways we uncover could be reactivated to promote remyelination in demyelinating diseases like MS or following stroke.
RESOURCES
DeActs plasmids available at Addgene:
Z-lab microscopes @FPbase:
"Mary Bartlett" Bruker Optera 2
 Find Brad Zuchero Lab Plasmids
Find Brad Zuchero Lab Plasmids